PharmaShots Weekly Snapshots (December 04 – December 08, 2023)
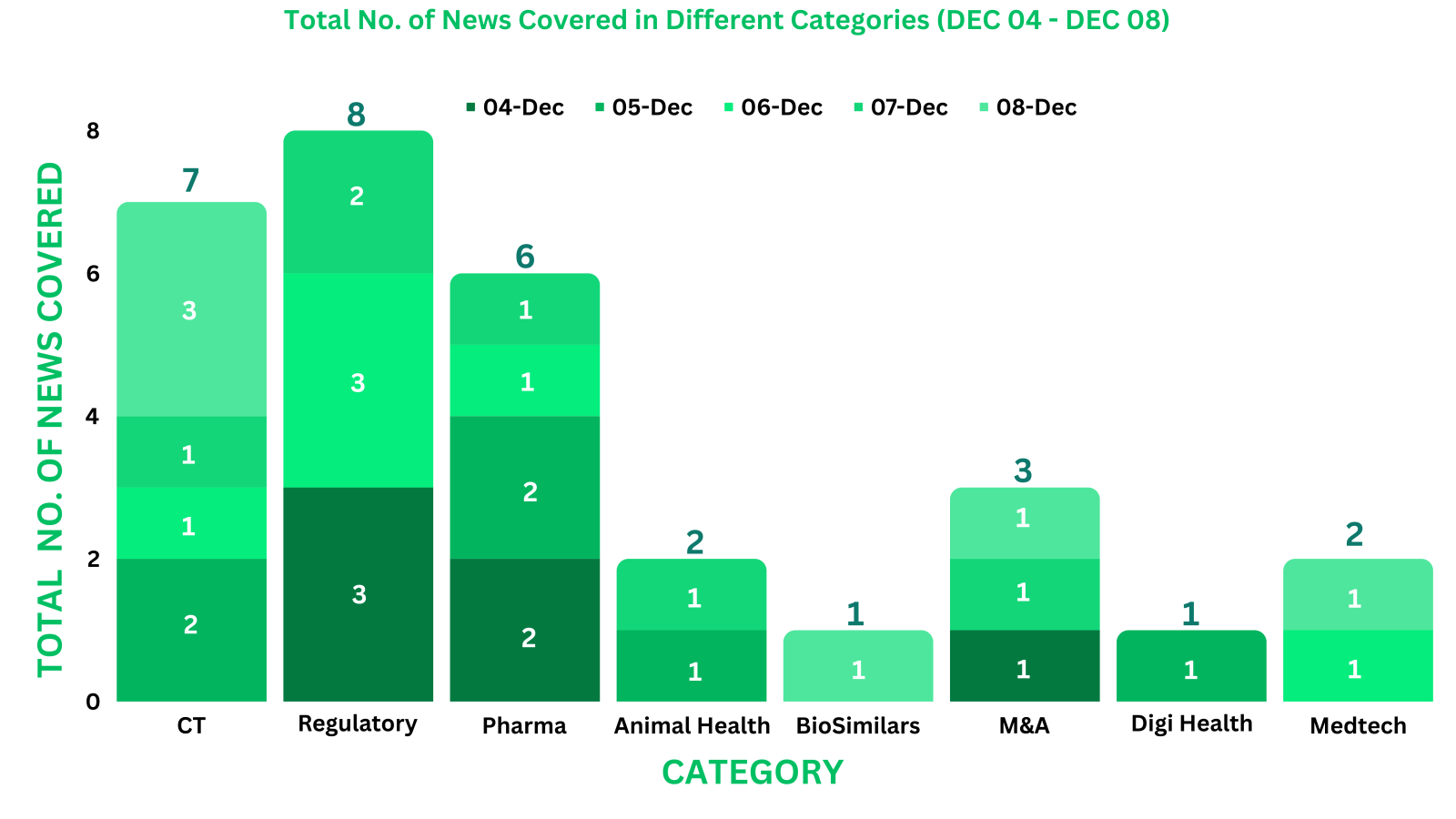
This week PharmaShots’ news was all about the updates on Pharma, Regulatory, Clinical Trials, DigiHealth, M&A & BioSimilar. Check out our full report below:

The NMPA Approves Everest Medicine's IND Application for Zetomipzomib
Read More: Everest Medicine
The TGA Accepts Shanghai Junshi’s Toripalimab for Treatment of Nasopharyngeal Carcinoma
Read More: Shanghai Junshi
The US FDA Approves Eli Lilly’s Jaypirca for the Treatment of Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma (CLL/SLL)
Read More: Eli Lilly
The US FDA Accepts BMS’s sBLA for Opdivo-Based Combination Therapy for Priority Review to Treat Urothelial Cancer
Read More: BMS
The US FDA has granted FTD and BTD to CG Oncology’s Cretostimogene Grenadenorepvec for High-Risk BCG-Unresponsive Non-Muscle Invasive Bladder Cancer
Read More: CG Oncology
The US FDA has Granted Approval to Novartis’ Fabhalta (iptacopan) for the Treatment of Paroxysmal Nocturnal Hemoglobinuria (PNH)
Read More: Novartis
BMS' Abecma has Obtained sNDA Approval in Japan for Early Lines of Therapy in R/R Multiple Myeloma (RRMM)
Read More: BMS
Sosei Group Secures MFDA’s Marketing Approval of Pivlaz (clazosentan sodium) for Aneurysmal Subarachnoid Hemorrhage (aSAH)
Read More: Sosei

Roche Announces the Acquisition of Carmot Therapeutics ~$3.1B
Read More: Roche
For About $8.7B, AbbVie to Acquire Cerevel Therapeutics
Read More: AbbVie & Cerevel Therapeutics
Vanda Pharmaceuticals Sings an Agreement with Janssen to Acquire the US and Canadian Rights for Ponvory from Janssen to Treat Relapsing Multiple Sclerosis
Read More: Vanda Pharmaceuticals, Janssen & Actelion

Nona Biosciences entered into collaboration with Lycia Therapeutics to develop Protein Degradation Technology
Read More: Nona Biosciences
Palvella Therapeutics and Ligand Pharmaceuticals are Expanding their Strategic Partnership to Expedite the P-III Development Of Qtorin Rapamycin
Read More: Palvella Therapeutics & Ligand Pharmaceuticals
Absci and AstraZeneca Sign a Collaboration Agreement to Discover AI-Driven Cancer Treatment
Read More: Absci & AstraZeneca
AQEMIA and Sanofi have Signed Multi-year Collaboration of $140M
Read More: AQEMIA & Sanofi
AbbVie Sings a Collaboration Agreement with BigHat Biosciences to Discover and Develop Therapeutic Antibodies
Read More: AbbVie & BigHat Biosciences
Innovent Biologics Expands its Licensing Agreement with Synaffix for the Development of Antibody Drug Conjugates (ADCs)
Read More: Innovent Biologics & Synaffix

The US FDA Renews Conditional Approval for Jaguar Health's Canalevia-CA1 for Chemotherapy-Induced Diarrhea in Dogs
Read More: Jaguar Health
Gallant Therapeutics is Set to Initiate the JEDI Study to Evaluate Allogeneic Stem Cell Therapy for Feline Chronic Gingivostomatitis (FCGS) in Cats
Read More: Gallant Therapeutics

GE HealthCare and AirStrip are going to Integrate their Expertise for Patient Monitoring and Cardiac Data Visualization
Read More: GE HealthCare & AirStrip
Clinical Trial
The US FDA Approves Tonix Pharmaceuticals’ IND Application for TNX-2900 to Treat Prader-Willi Syndrome
Read More: Tonix Pharmaceuticals
Genentech Highlights P-III Results for Inavolisib Combination Therapy to Treat Breast Cancer
Read More: Genentech
Merck KGaA Highlights the P-III Results for Evobrutinib to Treat of Relapsing Multiple Sclerosis (RMS)
Read More: Merck KGaA
Sanofi Highlights the P-III Trial Results for Sarclisa (Isatuximab) to Treat Newly Diagnosed Multiple Myeloma Patients
Read More: Sanofi
Merck will Discontinue its P-III (KEYLYNK-008) Study of Keytruda (pembrolizumab) + Lynparza (olaparib) for Metastatic Squamous NSCLC
Read More: Merck
The First Atopic Dermatitis Patient was Dosed with KT-474 in the P-II Trial Conducted by Sanofi Under its Collaboration with Kymera Therapeutics
Read More: Kymera Therapeutics & Sanofi
BMS' P-III (CheckMate -8HW) Study Evaluating Opdivo + Yervoy for MSI-H/dMMR mCRC has Achieved its Primary Endpoint
Read More: BMS

The US FDA Granted 510(k) Clearance to Exactech’s Surgical Navigation System ExactechGPS Ankle for Total Ankle Replacement
Read More: Exactech
The US FDA Approves Mallinckrodt’s INOmax EVOLVE DS Delivery System to Deliver Nitric Oxide Gas in Newborns with Pulmonary Hypertension
Read More: Mallinckrodt

The US FDA has approved Bio-Thera’s Avzivi (Biosimilar, Avastin)
Read More: Bio Thera
Related News:- PharmaShots Weekly Snapshots (November 27 – December 1, 2023)
Tags

Kritika is a content writer at PharmaShots. She is interested in covering recent innovations from the pharma & MedTech industry. She covers news related to Product approvals, clinical trial results, and updates. She can be contacted at connect@pharmashots.com.